Genetic Programming Theories Of Aging
Definition
The aging theories are a very loftier number, and also many scientists proposed different ways of dividing and categorizing them. Every bit a major subset of these theories, the genetic theories of aging include three chief concepts: (ane) the genetics of aging can be interpreted in the light of the evolutionary theories; (2) the genetics of aging and longevity can be informative if ecological and anthropological views are considered; (3) the genetics components underpin all the theories of aging even if not specifically stated.
Overview
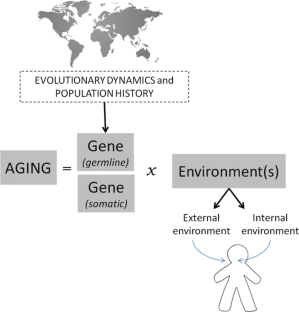
Aging and longevity (besides as each phenotype and circuitous trait) are the results of complex gene-environment interactions (GxE), and in modern humans, the dimension of the environment is very heterogeneous as it includes the cultural dimension and the ecological one (Giuliani et al. 2017). Moreover if nosotros consider the man body as the reference point, the term environment refers to the past and present environment and includes ii dimensions: (1) the...
References
-
Abondio P, Sazzini M, Garagnani P et al (2019) The genetic variability of APOE in different man populations and its implications for longevity. Genes 10:222. https://doi.org/10.3390/genes10030222
-
Alvergne A, Jenkinson C, Faurie C (eds) (2016) Evolutionary thinking in medicine. Springer International Publishing, Cham
-
Baines HL, Turnbull DM, Greaves LC (2014) Human stem jail cell aging: do mitochondrial Deoxyribonucleic acid mutations have a causal role? Crumbling Cell thirteen:201–205. https://doi.org/10.1111/acel.12199
-
Beirne C, Delahay R, Young A (2015) Sex differences in senescence: the part of intra-sexual competition in early adulthood. Proc R Soc B Biol Sci 282:20151086. https://doi.org/10.1098/rspb.2015.1086
-
Bonafè Grand, Olivieri F, Cavallone 50 et al (2001) A gender – dependent genetic predisposition to produce high levels of IL-six is detrimental for longevity. Eur J Immunol 31:2357–2361. https://doi.org/10.1002/1521-4141(200108)31:eight&#sixty;2357::AID-IMMU2357>three.0.CO;2-X
-
Bonafè Grand, Barbieri M, Marchegiani F et al (2003) Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and homo longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab 88:3299–3304. https://doi.org/10.1210/jc.2002-021810
-
Capri Grand, Santoro A, Garagnani P et al (2013) Genes of human longevity: an endless quest? Curr Vasc Pharmacol 12:707
-
Carter AJ, Nguyen AQ (2011) Antagonistic pleiotropy as a widespread mechanism for the maintenance of polymorphic disease alleles. BMC Med Genet 12:160. https://doi.org/ten.1186/1471-2350-12-160
-
Corella D, Ordovás JM (2014) Aging and cardiovascular diseases: the role of factor-diet interactions. Ageing Res Rev xviii:53–73. https://doi.org/10.1016/j.arr.2014.08.002
-
Corella D, Carrasco P, Sorlí JV et al (2013) Mediterranean diet reduces the adverse effect of the TCF7L2-rs7903146 polymorphism on cardiovascular risk factors and stroke incidence: a randomized controlled trial in a loftier-cardiovascular-chance population. Diabetes Care 36:3803–3811. https://doi.org/x.2337/dc13-0955
-
Crespi BJ (2011) The emergence of human being-evolutionary medical genomics. Evol Appl four:292–314. https://doi.org/ten.1111/j.1752-4571.2010.00156.ten
-
Dato S, Soerensen Thou, De Rango F et al (2018) The genetic component of homo longevity: new insights from the analysis of pathway-based SNP-SNP interactions. Crumbling Cell 17:e12755. https://doi.org/10.1111/acel.12755
-
Finkel T (2015) The metabolic regulation of aging. Nat Med 21:1416–1423. https://doi.org/x.1038/nm.3998
-
Fortney K, Dobriban East, Garagnani P et al (2015) Genome-wide scan informed by age-related disease identifies loci for infrequent human being longevity. PLoS Genet xi:e1005728. https://doi.org/10.1371/periodical.pgen.1005728
-
Franceschi C, Cossarizza A (1995) Introduction: the reshaping of the immune system with age. Int Rev Immunol 12:i–4. https://doi.org/10.3109/08830189509056697
-
Franceschi C, Monti D, Sansoni P, Cossarizza A (1995) The immunology of exceptional individuals: the lesson of centenarians. Immunol Today 16:12–sixteen
-
Franceschi C, Bonafè G, Valensin S et al (2000a) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
-
Franceschi C, Valensin S, Bonafè G et al (2000b) The network and the remodeling theories of aging: historical groundwork and new perspectives. Exp Gerontol 35:879–896
-
Garagnani P, Giuliani C, Pirazzini C et al (2013) Centenarians equally super-controls to assess the biological relevance of genetic run a risk factors for mutual age-related diseases: a proof of principle on type two diabetes. Aging 5:373–385
-
Garagnani P, Pirazzini C, Giuliani C et al (2014) The three genetics (nuclear DNA, mitochondrial DNA, and gut microbiome) of longevity in humans considered as metaorganisms. Biomed Res Int 2014:e560340. https://doi.org/10.1155/2014/560340
-
Garland T (2014) Trade-offs. Curr Biol 24:R60–R61. https://doi.org/ten.1016/j.cub.2013.11.036
-
Gavrilov LA, Gavrilova NS (2002) Evolutionary theories of aging and longevity. Sci World J two:339–356. https://doi.org/ten.1100/tsw.2002.96
-
Giuliani C, Pirazzini C, Delledonne M et al (2017) Centenarians as extreme phenotypes: an ecological perspective to get insight into the relationship between the genetics of longevity and age-associated diseases. Mech Ageing Dev. https://doi.org/ten.1016/j.mad.2017.02.007
-
Giuliani C, Garagnani P, Franceschi C (2018) Genetics of human longevity within an eco-evolutionary nature-nurture framework. Circ Res 123:745–772. https://doi.org/ten.1161/CIRCRESAHA.118.312562
-
Goldsmith TC (2017) Evolvability, population benefit, and the evolution of programmed crumbling in mammals. Biochem Mosc 82:1423–1429. https://doi.org/10.1134/S0006297917120021
-
Gorbunova V, Seluanov A, Mao Z, Hine C (2007) Changes in DNA repair during aging. Nucleic Acids Res 35:7466–7474. https://doi.org/10.1093/nar/gkm756
-
Govindaraju D, Atzmon Yard, Barzilai N (2015) Genetics, lifestyle and longevity: lessons from centenarians. Appl Transl Genom 4:23–32. https://doi.org/x.1016/j.atg.2015.01.001
-
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475:324–332. https://doi.org/10.1038/nature10317
-
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Jail cell 140:900–917. https://doi.org/10.1016/j.cell.2010.02.034
-
Houle D, Govindaraju DR, Omholt S (2010) Phenomics: the side by side challenge. Nat Rev Genet xi:855–866. https://doi.org/x.1038/nrg2897
-
Jaiswal S, Fontanillas P, Flannick J et al (2014) Age-related clonal hematopoiesis associated with agin outcomes. N Engl J Med 371:2488–2498. https://doi.org/10.1056/NEJMoa1408617
-
Kennedy BK, Berger SL, Brunet A et al (2014) Geroscience: linking crumbling to chronic disease. Cell 159:709–713. https://doi.org/x.1016/j.cell.2014.10.039
-
Kirkwood TBL (1977) Development of ageing. Nature 270:301–304. https://doi.org/ten.1038/270301a0
-
Kirkwood TBL, Holliday R (1979) The evolution of ageing and longevity. Proc R Soc Lond B Biol Sci 205:531–546. https://doi.org/10.1098/rspb.1979.0083
-
Koga H, Kaushik Due south, Cuervo AM (2011) Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev x:205–215. https://doi.org/10.1016/j.arr.2010.02.001
-
Lemaitre J-F, Berger V, Bonenfant C et al (2015) Early-late life trade-offs and the evolution of ageing in the wild. Proc R Soc B Biol Sci 282:20150209. https://doi.org/ten.1098/rspb.2015.0209
-
López-Otín C, Blasco MA, Partridge Fifty et al (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/ten.1016/j.jail cell.2013.05.039
-
Luis NM, Wang L, Ortega M et al (2016) Intestinal IRE1 is required for increased triglyceride metabolism and longer lifespan nether dietary brake. Cell Rep 17:1207–1216. https://doi.org/10.1016/j.celrep.2016.10.003
-
Martin GM, Oshima J (2000) Lessons from human being progeroid syndromes. Nature 408:263–266. https://doi.org/10.1038/35041705
-
Mitteldorf JJ (2012) Adaptive aging in the context of evolutionary theory. Biochem Mosc 77:716–725. https://doi.org/ten.1134/S0006297912070036
-
Pal S, Tyler JK (2016) Epigenetics and aging. Sci Adv two:e1600584. https://doi.org/ten.1126/sciadv.1600584
-
Parsons PA (1995) Inherited stress resistance and longevity: a stress theory of ageing. Heredity 75:216–221. https://doi.org/ten.1038/hdy.1995.126
-
Partridge Fifty, Gems D (2002) Mechanisms of ageing: public or private? Nat Rev Genet three:165–175. https://doi.org/x.1038/nrg753
-
Powers ET, Balch We (2013) Diversity in the origins of proteostasis networks – a driver for protein function in evolution. Nat Rev Mol Cell Biol xiv:237–248. https://doi.org/x.1038/nrm3542
-
Quintana-Murci Fifty (2016) Understanding rare and common diseases in the context of human evolution. Genome Biol 17:225. https://doi.org/10.1186/s13059-016-1093-y
-
Rando TA (2006) Stem cells, ageing and the quest for immortality. Nature 441:1080–1086. https://doi.org/x.1038/nature04958
-
Riera CE, Dillin A (2015) Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol 17:196–203. https://doi.org/10.1038/ncb3107
-
Rodríguez JA, Marigorta UM, Hughes DA et al (2017) Antagonistic pleiotropy and mutation accumulation influence human senescence and illness. Nat Ecol Evol 1:0055. https://doi.org/10.1038/s41559-016-0055
-
Su T, Turnbull D, Greaves L (2018) Roles of mitochondrial Dna mutations in stalk cell ageing. Genes 9:182. https://doi.org/ten.3390/genes9040182
-
Suh Y, Atzmon G, Cho M-O et al (2008) Functionally meaning insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci 105: 3438–3442. https://doi.org/ten.1073/pnas.0705467105
-
Ukraintseva Southward, Yashin A, Arbeev K et al (2016) Puzzling role of genetic risk factors in human longevity: "risk alleles" as pro-longevity variants. Biogerontology 17:109–127. https://doi.org/ten.1007/s10522-015-9600-1
-
Vaidya A, Mao Z, Tian X et al (2014) Knock-in reporter mice demonstrate that Deoxyribonucleic acid repair by non-homologous terminate joining declines with historic period. PLoS Genet 10:e1004511. https://doi.org/ten.1371/journal.pgen.1004511
-
van Exel E, Koopman JJE, Bodegom DV et al (2017) Effect of APOE ε4 allele on survival and fertility in an adverse environs. PLoS One e0179497:12. https://doi.org/10.1371/journal.pone.0179497
-
Van Meter Yard, Kashyap M, Rezazadeh S et al (2014) SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 simply this repression fails with stress and age. Nat Commun v:5011. https://doi.org/10.1038/ncomms6011
-
Zeng Y, Nie C, Min J et al (2016) Novel loci and pathways significantly associated with longevity. Sci Rep 6. https://doi.org/x.1038/srep21243
-
Zhang H-S, Chen Y, Fan 50 et al (2015) The endoplasmic reticulum stress sensor IRE1α in intestinal epithelial cells is essential for protecting confronting colitis. J Biol Chem 290:15327–15336. https://doi.org/10.1074/jbc.M114.633560
Author information
Authors and Affiliations
Corresponding author
Editor data
Editors and Affiliations
Department Editor information
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Giuliani, C., Garagnani, P., Franceschi, C. (2020). Genetic Theories of Aging. In: Gu, D., Dupre, M. (eds) Encyclopedia of Gerontology and Population Aging. Springer, Cham. https://doi.org/10.1007/978-three-319-69892-2_731-ane
Download citation
- .RIS
- .ENW
- .BIB
-
DOI : https://doi.org/10.1007/978-3-319-69892-2_731-1
-
Received:
-
Accepted:
-
Published:
-
Publisher Name: Springer, Cham
-
Print ISBN: 978-3-319-69892-2
-
Online ISBN: 978-3-319-69892-2
-
eBook Packages: Springer Reference Biomedicine & Life Sciences Reference Module Biomedical and Life Sciences
Genetic Programming Theories Of Aging,
Source: https://link.springer.com/10.1007/978-3-319-69892-2_731-1
Posted by: smiththerhave93.blogspot.com


0 Response to "Genetic Programming Theories Of Aging"
Post a Comment